Partnering for Combination Product Success: Higher-Level Collaboration that Delivers Higher ROI
Avoid the Combination Product pitfalls that derail timelines, budgets and market success. Misaligned vendors, regulatory gaps and siloed teams can cost you years and millions. Learn how to build the right internal and external partnerships from the start—and avoid a CRL.

Strategic Collaboration for Long-Term ROI and Regulatory Wins
Developing a drug delivery device system (combination product) adds a layer of complexity to your pharmaceutical program that many companies underestimate until it's too late. Success depends not just on technical execution but on strategic collaboration between internal departments, external partners and regulators. When misaligned, this ecosystem can stall launches, inflate costs, and jeopardize revenue.
This article outlines executive-level practical, proven approaches to:
- Stakeholder alignment
- Combination product planning
- Program execution
The goal is simple: enable you to invest your time, teams and capital in the right places the first time, before setbacks become crises.
The Executive Challenge
Combination products require integrating two very different development paradigms: the long, linear drug development timeline and the comparatively fast, iterative device development process. These paths must be coordinated in a way that satisfies regulators and drives commercial success, without creating delays or redundancy.
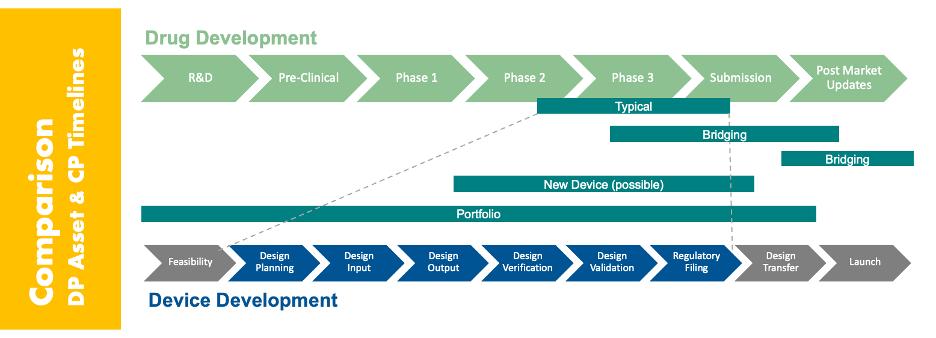
Complicating this are three realities:
- Functional teams inside your company often lack familiarity with each other's processes.
- Device-related activities introduce new regulatory, quality, and human factors requirements that most pharma teams haven’t worked with before.
- External partners (OEMs, CMOs, CROs, suppliers) must work in lockstep with your internal systems and strategy in ways that feel unfamiliar to a pharmaceutical company.
The result? A multidirectional web of communication and dependency (depicted below), often without a clear owner or cohesive strategy—unless you proactively design one.
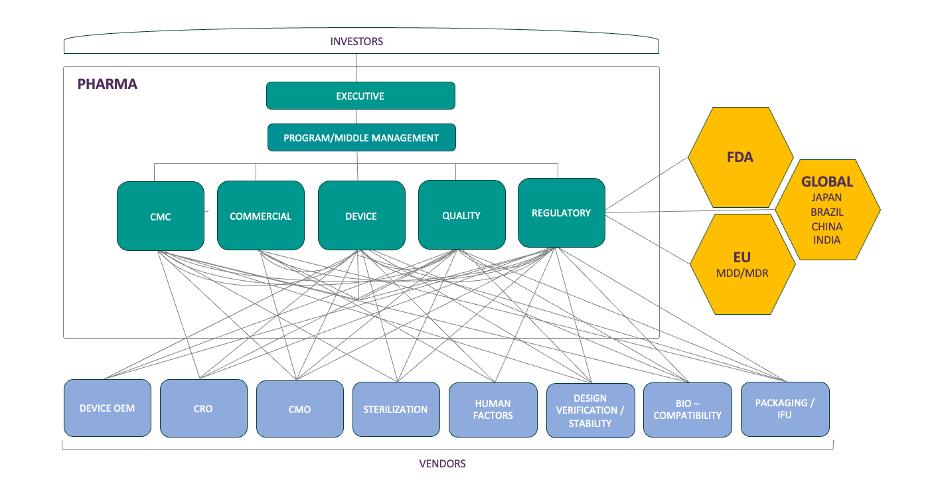
Your Best Asset: A Dedicated Combination Product Integration Team
Whether in-house or outsourced, a dedicated team that acts as the connective tissue across departments and partners can de-risk your development program. These subject matter experts act as system engineering architects to bring together the various sub-systems of your delivery device into an approvable combination product. Their job is to:
- Ensure the technical integration of the various constituents is robust (e.g. how does the selection and variability of your primary container impact the system function when paired with the device constituents).
- Ensure alignment with the CMC organization on timelines, regulatory plans and risk management strategies and where key milestones plug in from the device development activities.
- Translate between "drug language" and "device language."
- Implement systems and processes that facilitate clear, proactive communication. Both to meet the business needs as well as the quality system needs related to managing a combination product.
This role isn’t just administrative—it’s strategic. When well-executed, it reduces program drag, uncovers risks early and enables more confident decision-making at every level. It helps remove the unknown unknowns in your business and proactively identify technical and program risks that may derail your approval and launch.
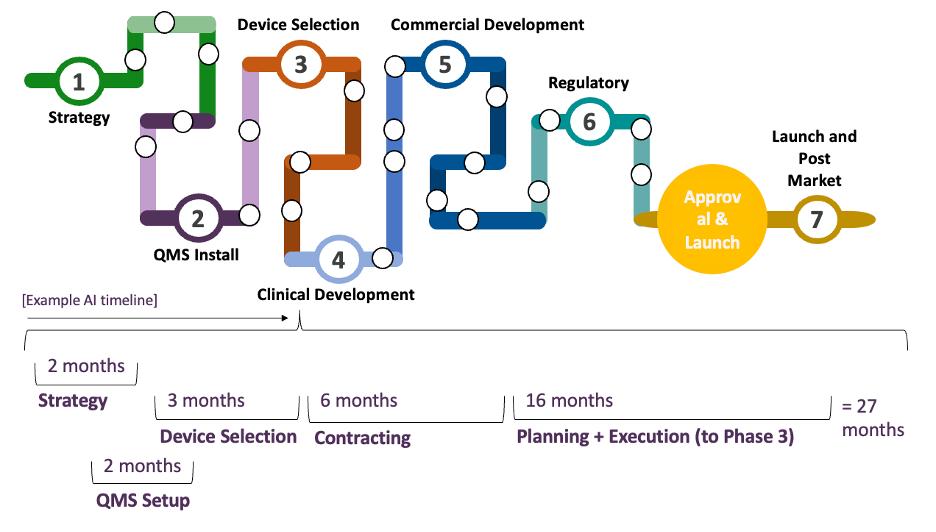
Organizing Complexity: The Seven-Phase Framework
To manage the complexity of combination product development, we organize the work into seven interrelated (but not strictly linear) phases. This framework helps teams focus, align and identify critical touchpoints between functions.
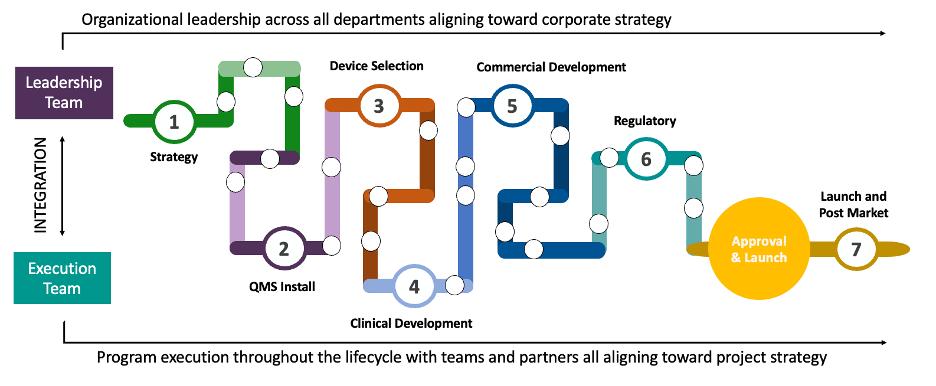
1. Strategy
Develop an integrated product and portfolio strategy that aligns regulatory pathways, commercial goals, device platform flexibility, vendor responsibilities, and budget constraints. Ensure risk and timeline assessments are realistic and account for potential uncertainties throughout development.
2. QMS Install
Right-size your Quality Management System by tailoring SOPs to fit your organization’s scale and complexity. Focus on customizing key elements such as Design Controls, Risk Management, and Purchasing to ensure compliance and efficiency.
3. Device Selection
Align selection and vendor management with your clinical and commercial strategy. Define cross-functional criteria and futureproof decisions.
4. Clinical Development
Design and execute a phase-appropriate clinical program that balances risk management with development goals without overcomplicating early clinical activities. Early-stage clinical work should be strategically aligned to support long-term commercial development.
5. Commercial Development
Align commercial-phase activities with the intended use of your product by finalizing design, labeling, IFU, packaging, and any post-clinical updates. Ensure human factors validation, process validation and overall readiness for a successful submission and launch.
6. Regulatory Execution
Lead interactions with the FDA (e.g., Type C, Pre-IND) and submission readiness. Avoid assuming vendors will hand you submission-ready content.
7. Launch & Postmarket
Map post-launch responsibilities: reimbursement, training, adverse event reporting, and updates to your risk and quality systems. In each phase, clear connection points across functions, and across companies must be deliberately managed.
Case in Point: When Poor Alignment Costs Millions
Recently, we were brought in to support a mid-sized pharma company already partnered with a device OEM (Original Equipment Manufacturer) to launch a combination product. Despite prior experience with similar combination products, they were encountering numerous challenges including:
- Regulatory delays: Despite prior success, this submission received a device related CRL (Complete Response Letter)
- Budget overruns: Due to missed testing/retesting
- Timeline slippage: As the challenges mounted, timelines suffered
- Investor frustration: The view of “It’s just a device – how complicated can it be” began to set in with growing frustration across the leadership and investors.
Following review of various elements of the Design History File, our assessment revealed:
- Gaps in Risk Management and Design Control documentation: Specifically, oversights that date back to earlier decision making on the program.
- Lack of cross-functional accountability: Including clear lines of communication and alignment on roles and responsibilities across the device team with other divisions of the company (CMC Project Management, Regulatory Affairs, Quality Assurance, etc.).
- Misaligned regulatory assumptions: What the regulatory teams stated as their strategy was misaligned with what the development team was executing against and documenting within their DHF
- Vendor documentation shortfalls: The absence of a trust-but-verify approach resulted in gaps between the client documentation and their vendors and painful oversights caught in the 11th hour by reviewers.
- Disconnected quality systems: A lack of integration across the core pharmaceutical QMS and the device QMS was causing redundancy and creating regulatory and quality risks for the program.
Below is a grid that shows the cross-functional impact of these issues.
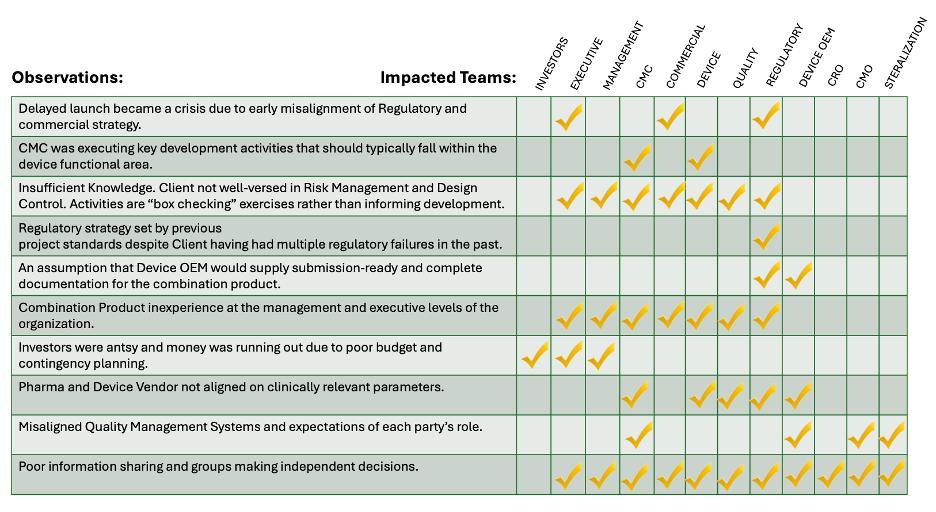
The cost of the delay? Nearly $1.6 billion in lost and deferred revenue and value across the supply chain (including $520M in first-year pharma revenue, $100M for the CMO, and more for the device and service vendors). Our intervention helped cut the delay from 24 to 18 months—but earlier alignment could have avoided the issue entirely.
Key takeaway for executives: A delay caused by misalignment isn’t just a project risk; it’s a business risk. Strategic preparation is cheaper than remediation.
Strategic Solutions for Prevention
1. Start with Early Cross-Functional Inputs
Every function—from commercial to clinical to quality and manufacturing—needs visibility into early device development. Dependencies aren’t linear. Learning of a new requirement late into the development process can have drastic impacts on the program and early design decisions. Aligning on things like user needs, clinical settings, manufacturing assumptions and timelines before jumping into the development process can have significant returns over the long run of the program, avoiding costly and timely rework. These decisions should be formally documented in your programs planning documents, design reviews and reports. This ensures a clear consensus on the path forward.
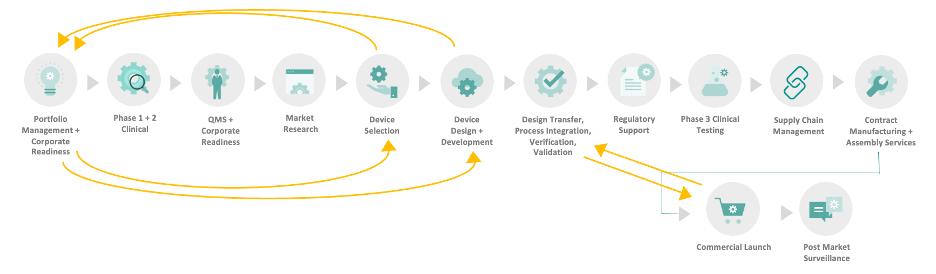
2. Map Phase-by-Phase Partnership Touchpoints
Strategic planning requires:
- Defining the regulatory pathway early and socializing it across the entire team.
- Right-sizing your QMS before you select a device.
- Understanding how clinical and commercial strategies diverge and aligning risk and HF work accordingly.
- Linking product value proposition to device design choices.
- Setting realistic and achievable development timelines (see below for an example autoinjector development timeline)
3. Build the Right Team
While the below functions may roll up under different divisional leaders, your core team should include:
- Device Engineering: Often times engineers with a system engineering background who understand how to link together multiple sub-systems into a final combination product. Depending on the complexity of your device, this may be further sub-divided into expertise on testing, design or software.
- Project Management: Helping steer the program and navigate the various roadblocks. Documenting and escalating key decision points in a timely manner.
- Device Quality: Playing a key role in ensuring compliance with applicable procedures and in many cases supporting the team in the navigation of global quality processes like CAPA, Change Control, etc.
- Device Regulatory: Helping identify the correct regulatory strategy as well as applicable FDA precedence, guidance and interactions to improve your products chances of regulatory approval.
And they must work fluidly with Regulatory CMC, Supply Chain, Manufacturing, Commercial, Clinical, Medical Affairs and others. Hiring for direct experience—not theoretical knowledge—can be the difference between a smooth launch and a surprise CRL.
4. Redefine Your Vendor Relationships
For traditional pharmaceutical companies used to treating vendors as task-doers, redefining those relationships may feel uncomfortable at first. However, the reality is that in the device world many of your vendors need to fill the role of a strategic partner. Due to constraints around IP, expertise or capabilities, your vendor relationship will often own major aspects of your Design History File or Risk Management File and your systems/documentation will need to be integrated seamlessly to ensure smooth coordination. This requires alignment on some of the following key elements before any development work is even started:
- Technical expectations
- Documentation processes
- Shared QMS responsibilities
- Communication protocols
- Submission deliverables
- Future supply and lifecycle planning
And yes—formalize the above alignments in contracts, risk plans, and meeting cadences. Click here for more on this subject.
Practical Action Plan for Executives
Navigating the complexity of combination product development isn’t about checking boxes—it’s about proactively building an ecosystem that’s aligned from the start. Here’s how executive teams can translate strategic intent into operational execution:
Priority | Action |
Invest Early in Integration Leadership | Appoint or engage a dedicated combination product integration team. Ensure they have the mandate and authority to bridge internal silos and manage external partner alignment. This team should have both technical fluency and strategic oversight. |
Operationalize Cross-Functional Input | Formalize early collaboration across Regulatory, Clinical, CMC, Quality, Commercial, and Supply Chain. Codify assumptions, dependencies, and constraints in planning documents and design reviews. What’s unspoken early becomes expensive later. |
Use a Phase-Based Operating Model | Adopt a structured framework—such as our Seven-Phase Model—to align workstreams, identify key decision points, and build accountability in every phase. Use it as a living program management tool, not just a conceptual reference. |
Reframe Vendor Engagement as Strategic Partnership | Treat vendors not as outsourced executors, but as co-owners of your program’s success. Establish shared expectations, documentation standards, and governance structures up front. Build mutual visibility and traceability from day one. |
Prioritize Regulatory and Quality Systems Readiness | Align your QMS and regulatory strategy with device-specific requirements early. Don’t retrofit compliance—design for it. Ensure internal teams and vendor systems are integrated and audit-ready ahead of submission. |
Final Thought
Combination product success is never accidental. It’s built through intentional collaboration, aligned incentives, and experienced leadership.
If you’re in Phase 2, now is the time to set the structure that will carry you through approval and into the market. Strategic investment today protects your timeline, your budget, and your patients tomorrow.
For more on Vendor Management, click here.
To pick our brain on partnering for success, click here or email discuss@suttonscreek.com
Speakers